Description
(MgCl2 x 6H2O)
Addition according to the combined magnesium chloride and sodium bicarbonate dosage.
Best practice:
By appling this method Magnesium and Carbonates where supplemented to the aquarium water, by adding hw®-Magnesiumchlorid (MgCl2*6H2O) and hw®-Natriumhydrogencarbonat (NaHCO3). As excess components Cl (chloride) and Na (sodium) will remain and the end result is NaCl = salt. NaCl is one of the greatest components of natural marine water but a steadily increase of NaCl will lead to a negative change in the water composition.
To avoid such changes it is useful to compensate the increasing NaCl component by applying a NaCl „free“ marine salt (hw®-MineralMarin). By this way the end result will be again normal marine water, without changing the ion balance of the marine water.
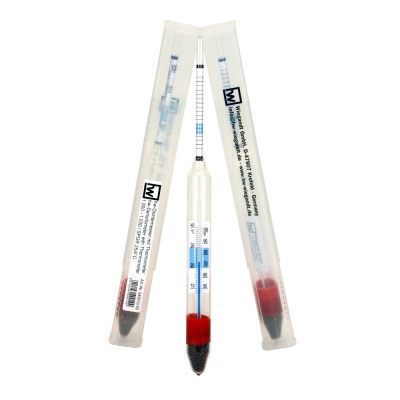
Only side effect of this method is, that the density / salinity of the aquarium water will slowly increase. You can control this side effect by measuring the Density / specific gravity from time to time with a hw®-Densitometer with Thermometer. If you notice an increasing of the measured value you can compensate this by just only adding a higher part of fresh water when making the next water change.
Addition in accordance to the Magnesium sulfate and Magnesium chloride dosing method.
The main concept:
Beside sodium chloride, magnesium is the second biggest component of the natural marine salt. But compared to sodium chloride, the magnesium level in the aquarium water will slowly declaim due to the adsorption of several marine organisms (like corals, mollusks and other reef building organisms) and bio-chemical processes. Therefore to avoid deficits in Magnesium the concentration of this element should be adjusted from time to time. In natural marine water the Magnesium concentration is around 1250 – 1380 mg/l and depends strongly from seasonal changes and geographic location.
To elevate specifically the Magnesium concentration in the aquarium water you will need hw®-Magnesiumchlorid-Hexahydrate and also a specified amount of hw®-Magnesiumsulfat-Heptahydrat, to not alter negatively the natural balance of the chloride- and sulfate ions in the your aquarium water.
Best practice:
The exact calculation of the needed quantities in gram has to be made by calculating with the molar mass and under consideration of the bounded crystal water together with the fixed relation of chloride to sulfate in natural marine water. By using the combination of hw®-Magnesiumchlorid-Hexahydrate and hw®-Magnesiumsulfat-Heptahydrat the weight relations are as following:
0,95 g hw®-Magnesiumsulfat-Heptahydrat (MgSO4x7H2O)
7,58 g hw®-Magnesiumchlorid-Hexahydrate (MgCl2*6H2O)
_____________________________________________________
1,00 g Magnesium (Mg)
Sample calculation:
A marine aquarium with 500 l water volume has a Magnesium concentration of 1200 mg/l, the concentration should be elevated to 1300 mg/l. To calculate the needed quantity of Mg in gram proceed as following:
1300 -1200 = 100 mg/l * 500 l (aquarium volume) = 50 g Mg in total
50 g x 0,95 g = 47,50 g hw®-Magnesiumsulfat-Heptahydrat (MgSO4x7H2O)
50 g x 7,58 g = 379,00 g hw®-Magnesiumchlorid-Hexahydrate (MgCl2*6H2O)
Both quantities should be solved together in around 600 ml RO-water (or demineralized water) and filled up to finally 1000ml. From this solution should be added 100 ml daily over the next 10 days to the aquarium water. On the 5. day please realize a control measuring of the actual magnesium level/concentration in the aquarium water, so that eventually adaptation could be made.