Why or when do I need a hw reverse osmosis system?
If the tap water contains too high degrees of hardness (KH / GH) and / or too many pollutants (nitrates, phosphates, silicates, heavy metal compounds etc.) as that this levels could be tolerated by the delicate marine organism in your reef/marine aquarium.
Many substances in your tap water, which are totally safe for humans in the present concentrations, are extremely harmful for reef organisms or can causes unforeseen disturbances in the biological system of the aquarium.

How freshwater becomes seawater …
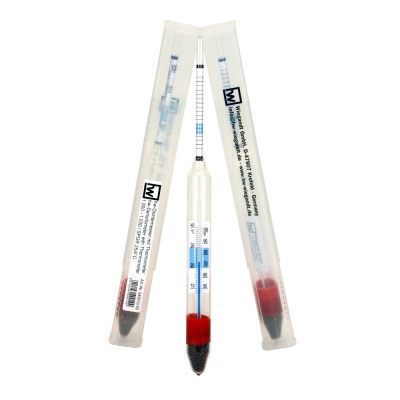
For this you need pure water (ideally from a hw reverse osmosis system) and hw-Marinemix professional or hw-Marinemix reefer. You also need a “solution container” (plastic barrel), a small submersible pump (works without it, but then you will have to stir the water by hand), a thermometer, a density meter (better still a density meter with thermometer and temperature compensation information, such as the hw® density meter with thermometer) or a refractometer for density or salinity determination. Fill the barrel with water (up to approx. 10 cm below the edge of the barrel). Now place the pump in the barrel. Connect it and measure the temperature of the water. Why the temperature? This has something to do with the dissolving behavior of salts, because if the water is too cold (below 20 ° C), the salts of the sea salt mixture dissolve significantly slower and not so easy than in “warm” water. This is not a quality matter of the used marine salt mixture, this is only caused through the physical conditions in the solution processes. If the water is too cold, leave everything at room temperature until the next day or use an aquarium heater until water temperature of at least 20 ° C is reached. Now you can start to dissolve the hw-Marinemix in the water. Our tip: Always dissolve only small amounts of marine salt, wait until the salt has completely dissolved before adding new ones to the water. Check the density / salinity from time to time to ensure that the desired value has not already been reached. Remember, you can still add a little bit of marine salt, but what is added too much cannot be removed (you can only try to add more fresh water, if your container is not filled up to the top).
Another general rule for the dissolution of sea salt mixtures in water: Always rinse the marine salt in the already water filled container, NEVER fill water on top of the marine salt! If you let water flow onto the marine salt, a completely supersaturated solution is created immediately. The result will be precipitations in the form of water-insoluble carbonate compounds.
What is “Specific Water Density”?
In marine aquariums, the specific water density is synonymous with the “salinity” of the water. The higher the specific density, the more salts are dissolved in the water. However, these measurement methods are very temperature-dependent. Basically for water, like for other substances, as the temperature rises, the molecules move more and more violently and thus take up more and more space. Therefore, the substances usually expand with increasing temperature, their density decreases. This means that 30 ° C warm water (due to the larger volume) has a lower, measurable specific density than the same water at 15 ° C or 20 ° C. If you work with an aerometer (density meter), pay attention to which reference temperature it is set to! If such information cannot be found on the aerometer, it is better not to use it as a “measuring device”.
What is salinity?
The salinity reflects the proportionate salinity of the water in parts per thousand (‰) and cannot be influenced by the water temperature in the measuring process. It is therefore the “more correct” method when it comes to determining reference and comparison values. However, this only works if the refractometer used is specially designed for sea water. Otherwise there are constant measurement errors. Sea water has an average salt content (salinity) of 3.5% by mass or more precisely 35 ‰ for our applications. This corresponds to a salt content of 35 grams per kilogram of sea water. The total salt content fluctuates depending on the sea and sea region. For this reason, the water values should be based on the home regions of your fishes an corals if possible.
Why should I wait 1-2 hours after dissolving the marine salt?
From a purely chemical point of view, sea water is a very aggressive lye (it is not for nothing that also “stainless” steel decomposes in sea water).
By dissolving marine salt numberless chemicals reactions are involved in the solution process, which should be completed before the so prepared water should be applied to the aquarium.
Why wait?
Well, we all know that the reaction of hydrogen and oxygen results in water … You can drink that water, but do you want the reaction (chemistry experiment “detonating gas test”) to take place in your mouth? It is the same with our aquarium inhabitants, they love the fresh sea water when doing water changes, but they are surely not interested in the chemical reactions of a still not finished solution processes.